More good news for Theranos and bad news for blood-testing centers run by hospitals, clinics, private blood-taking-and-testing labs and physician groups.
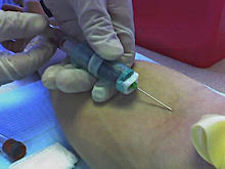
The Food and Drug Administration has approved Theranos’s new finger-prick method, which requires mere drops of blood as opposed to vials to run complex tests.
It has also approved Theranos’s request to conduct, for starters, a herpes test outside of a lab — a so-called CLIA waiver. This means, of course, that the agency is letting the Palo Alto-based startup offer consumers greater flexibility and itself a broader revenue stream, which would flow away from current traditional blood takers and testers.
USAToday says that “Possible implications of a CLIA-waver would be creating a test that could be taken at home, akin to a pregnancy test, while another would be to allow patients to get immediate test results at Theranos’s growing network of Wellness Centers without having to export the blood work to one of its labs.”